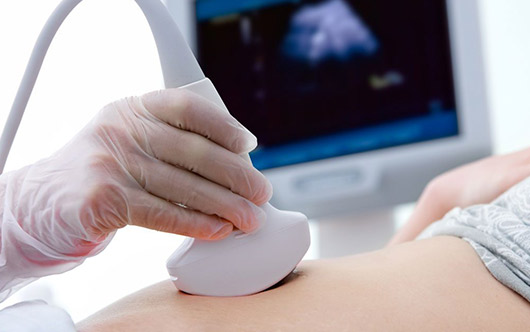
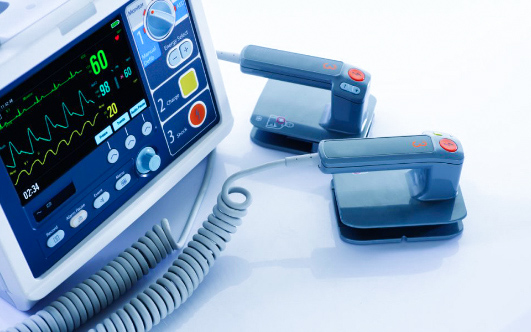
Quality, processes and traceability are key factors of a successful medical product. Norautron support customers at each stage of their product lifecycle with a relevant footprint of ISO 13485 certified facilities. We focus on high mix to low/medium volume projects, putting our expertise and approach to regulatory compliance standards. We work closely with our customers on product development and offer our knowledge on quality assurance and regulatory requirement that are specific to medical devices.